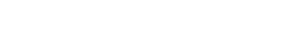 Image by: Matthew Newby SWNS
Image by: Matthew Newby SWNS
The family of a baby boy given three months to live are desperately waiting to see if the NHS will pay for the drug that could save his life.
Six-month-old Haris Khan was born with a rare and debilitating genetic disorder, Spinal Muscular Atrophy (SMA), also known as floppy baby syndrome.
When his family were given the devastating diagnosis this month they were also delivered the mortifying blow that the drug that could give him a better, longer life had been pulled in November last year.
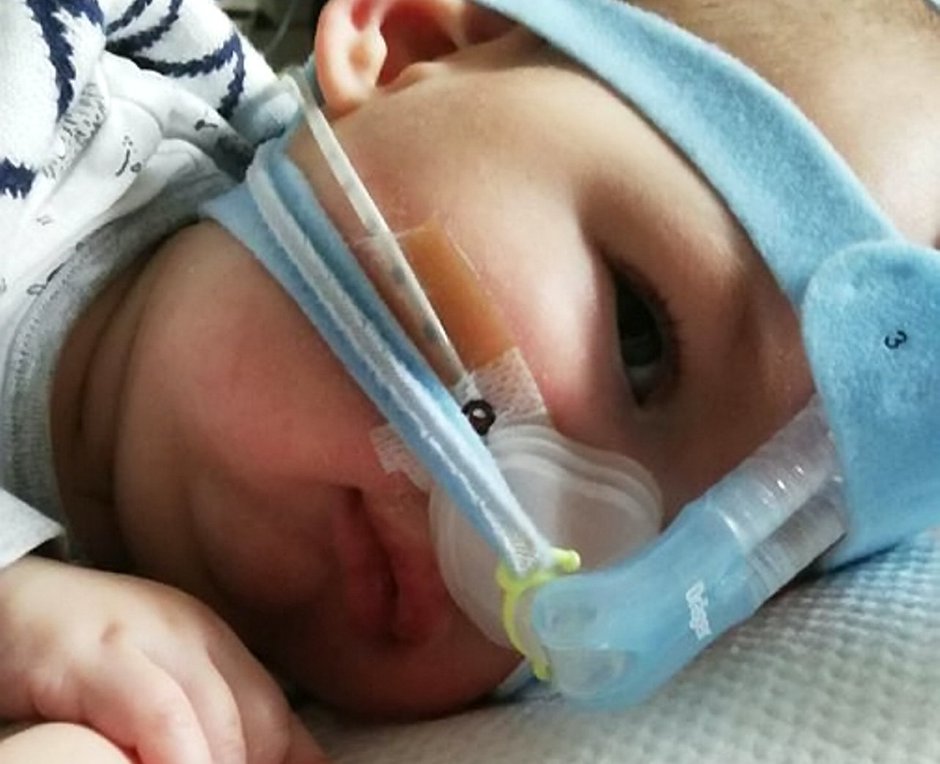
Now, Haris’ dad, salesman Shakeel, is joining a protest next month – along with other SMA families – to fight for the £450,000-a-year drug, Spinraza.
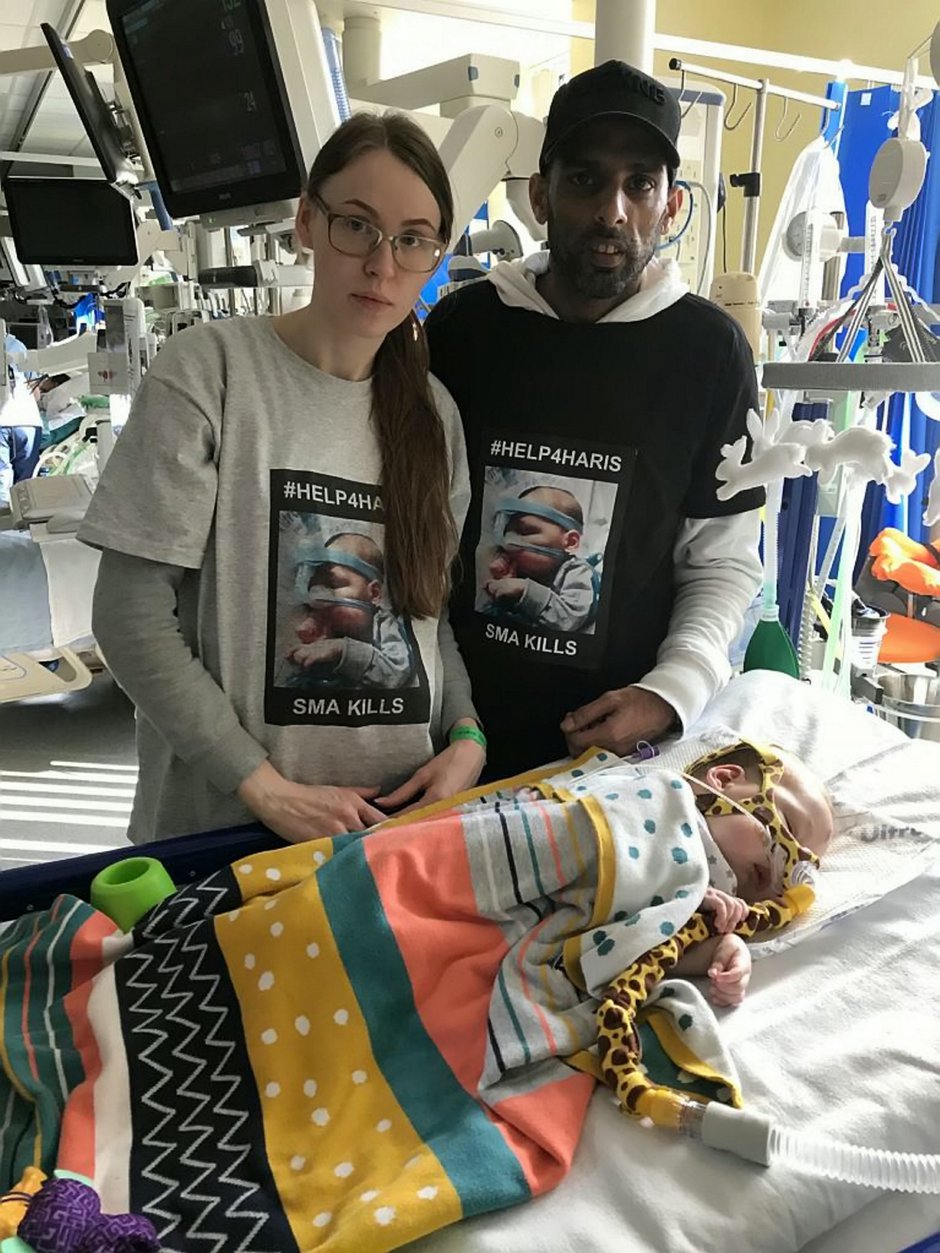 Image by: Matthew Newby SWNS
Image by: Matthew Newby SWNS
They will be gathering outside the NICE offices in Manchester next Tuesday ahead of a crucial meeting to come to an agreement over funding.
In the meantime, adorable Haris, from Wythenshawe, Greater Manchester, is in intensive care at the Royal Manchester Children’s Hospital attached to an apparatus that helps him breathe.
His family of 31-year-old dad, mum, shop assistant Renata, 26, and nine-year-old brother Marijus, are staying at a local hotel so they can be at Haris’ bedside 24/7.
They fear each moment could be his last.
On February 14, specialist doctors told the family Haris has the severest form of the illness, type 1, which affects the nerve cells needed to control the muscles we use for moving, swallowing and breathing.
The irony is, that if Haris had been diagnosed sooner – he was born in August last year – he would have already been given the drug, as it available on a pre-approval basis.
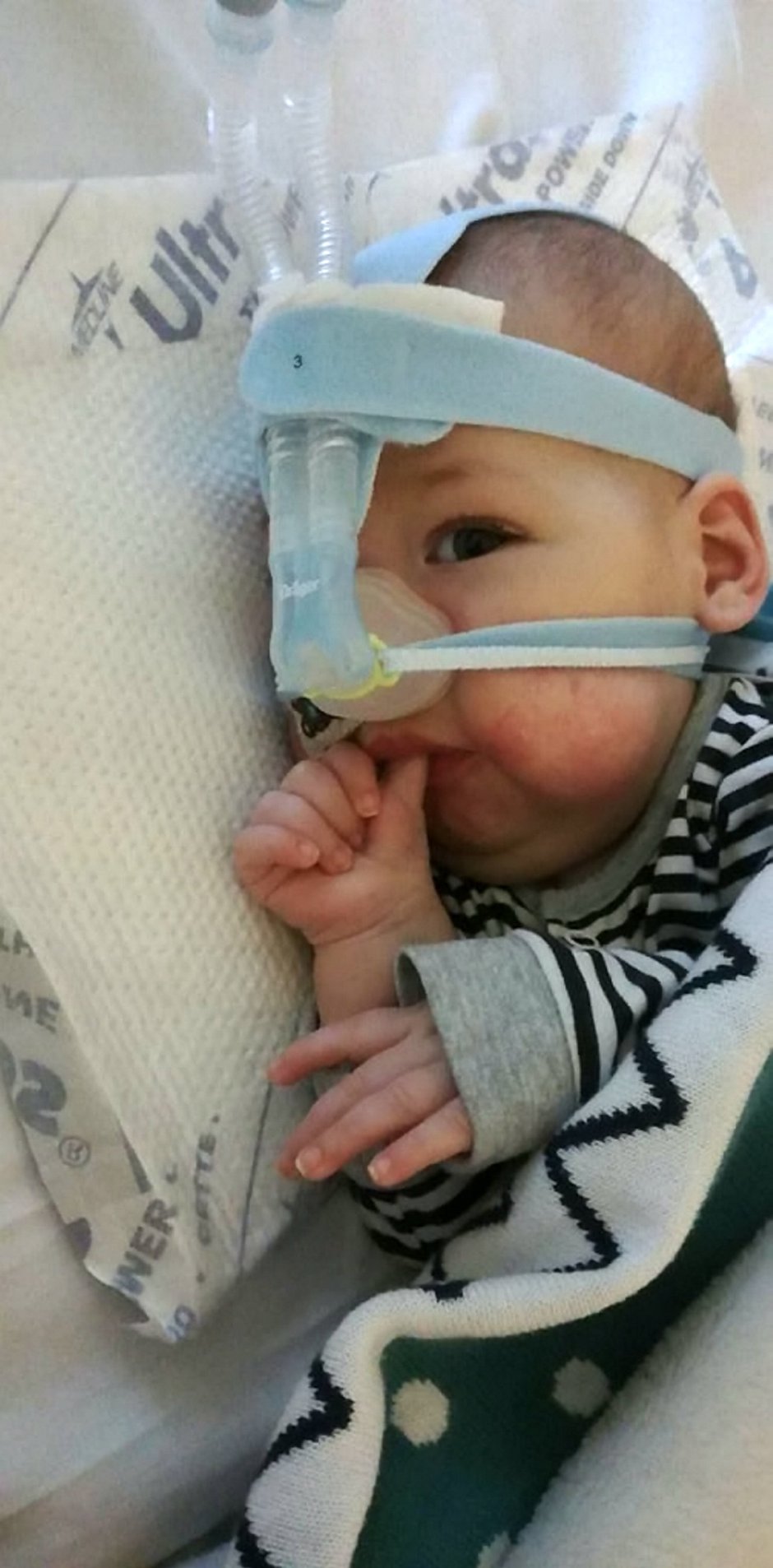 Image by: Matthew Newby SWNS
Image by: Matthew Newby SWNS
The first British girl to receive it, Annabelle Rose Thomas, has come off breathing support, can swallow food and has even ridden a horse.
But last summer, the National Institute for Health and Care Excellence (NICE) said it was not recommending Spinraza be available on the NHS in England because the cost was deemed ‘too high for it to be considered a cost-effective use of NHS resources’.
It remains available in Scotland and several other European countries.
Biogen, the company which makes the drug, charges almost £600,000 for it in the US market.
It is offering Spinraza at a lower price of £450,000 for the first year in the UK, and offered an undisclosed discount to the NHS, but it was still not enough.
Shakeel said: “Only a hundred babies a year maximum need that treatment. We, as a country, are losing a hundred babies because we don’t think it’s justified to save them.
“These organisations are using my son as a bartering tool. The best I can do is to save my son, or keep him with us for as long as possible.
“I’m just beginning my journey in parenthood – I want him here as long as he can be.
“There is a one in four chance my next child will have this – we can’t have any more children.”
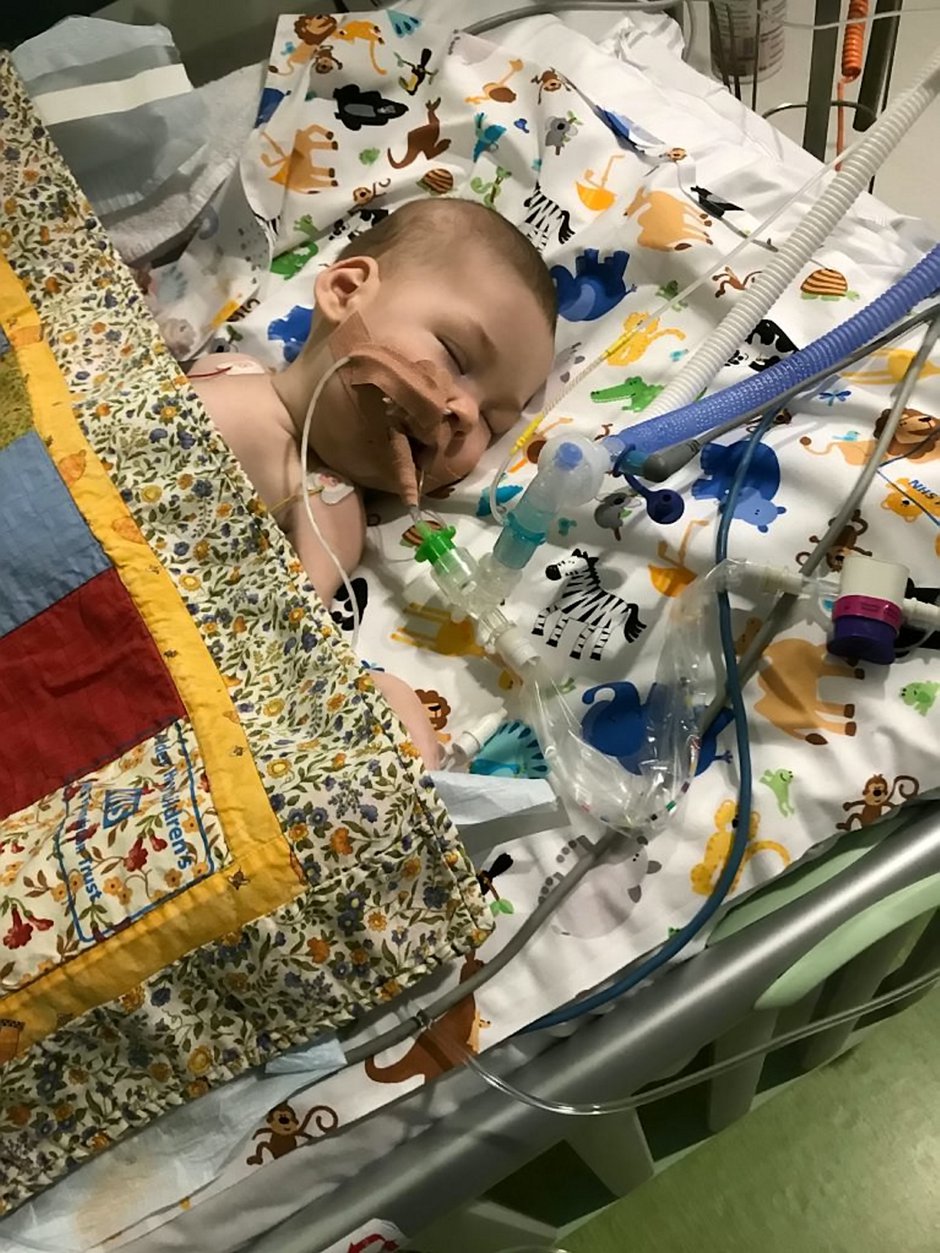 Image by: Matthew Newby SWNS
Image by: Matthew Newby SWNS
Amazingley, the Wythenshawe community had shown tremendous support for the Khan family.
Every neighbour on their street has a poster in the window about SMA.
Marijus’ school and the Rainbow Trust Children’s Charity are helping the family any way they can.
Whatever the outcome for Haris and the decision on March 6, Shakeel and Renata don’t want other families to go through the same ordeal.
“We might only have my Haris for a few months, so I’ve got to create a legacy for him,” said Shakeel.
“The NHS don’t screen for SMA at birth.
“Had they screened him in August when he was born, the treatment was available until November on the Early Access Programme.
“Eighty infants are on that – Haris would have been 81.
“That’s one thing we’re calling for.”
Haris is the youngest child with SMA to feature on a leaflet being handed out to all MPs this week by the charity Muscular Dystrophy UK.
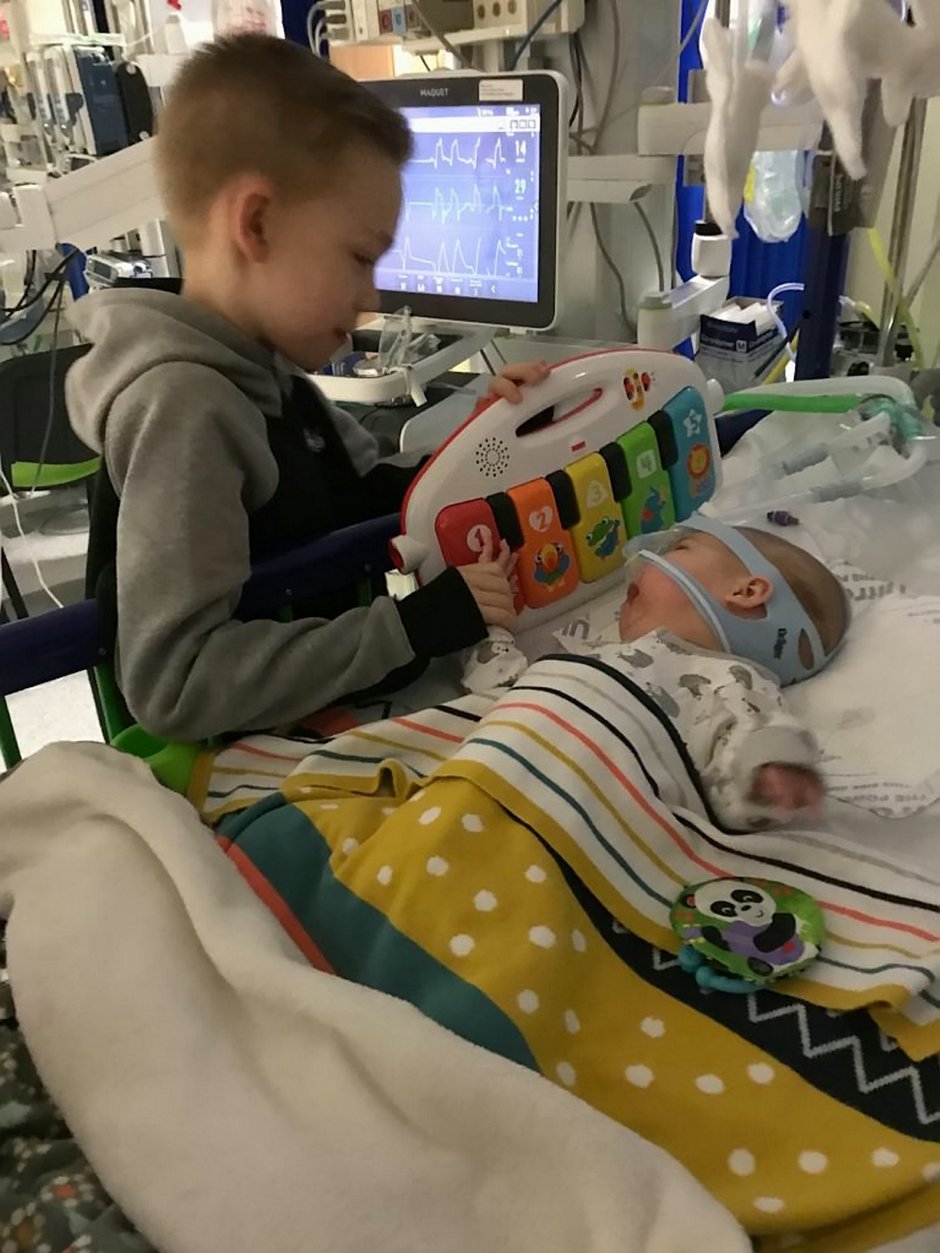 Image by: Matthew Newby SWNS
Image by: Matthew Newby SWNS
Shakeel wants as many people as possible to be aware of the fight and support the cause.
“Every SMA parent is waking up thinking ‘is this our child’s last day?’ he said.
A spokesperson for NHS England said: “We understand how difficult and frustrating it is for families waiting for decisions to be taken on the funding of new treatments, which is why the company must price this drug responsibly and at a level which is both cost effective and affordable to the NHS.
“It is disappointing that Biogen chose to close the Early Access Programme for new patients with Spinal Muscular Atrophy Type 1 before the NICE assessment process had been completed.”
A JustGiving page has been set up to raise money to support the Khan family’s campaign to improve awareness of SMA. It is also where Shakeel is posting updates on Haris and his battle. You can visit and donate here: https://www.justgiving.com/crowdfunding/littleharis?utm_id=69
Video by: Matthew Newby SWNS